The Projecting Subcutaneous and Self-Injectable Use Model is a web-based tool developed by Track20 to estimate the number of potential subcutaneous injectable (SC) and self-injectable (SI) users annually through 2030 for low and lower-middle income countries.
Analysis briefs for Burkina Faso, Kenya, Malawi, Nigeria, Pakistan, Senegal and Uganda are available at the bottom of the page.
 Access the Projecting Subcutaneous and Self-Injectable Use Model Here
Access the Projecting Subcutaneous and Self-Injectable Use Model Here

Understanding the potential market for SC and SI users is important for family planning programs. The model presented here takes country specific information about the family planning environment to inform the growth opportunities of injectables and allows users to change key parameters to see the effects of different levels on increasing SC use. Additionally, the model allows for the separation of provider injected subcutaneous injectables and self-injectables.
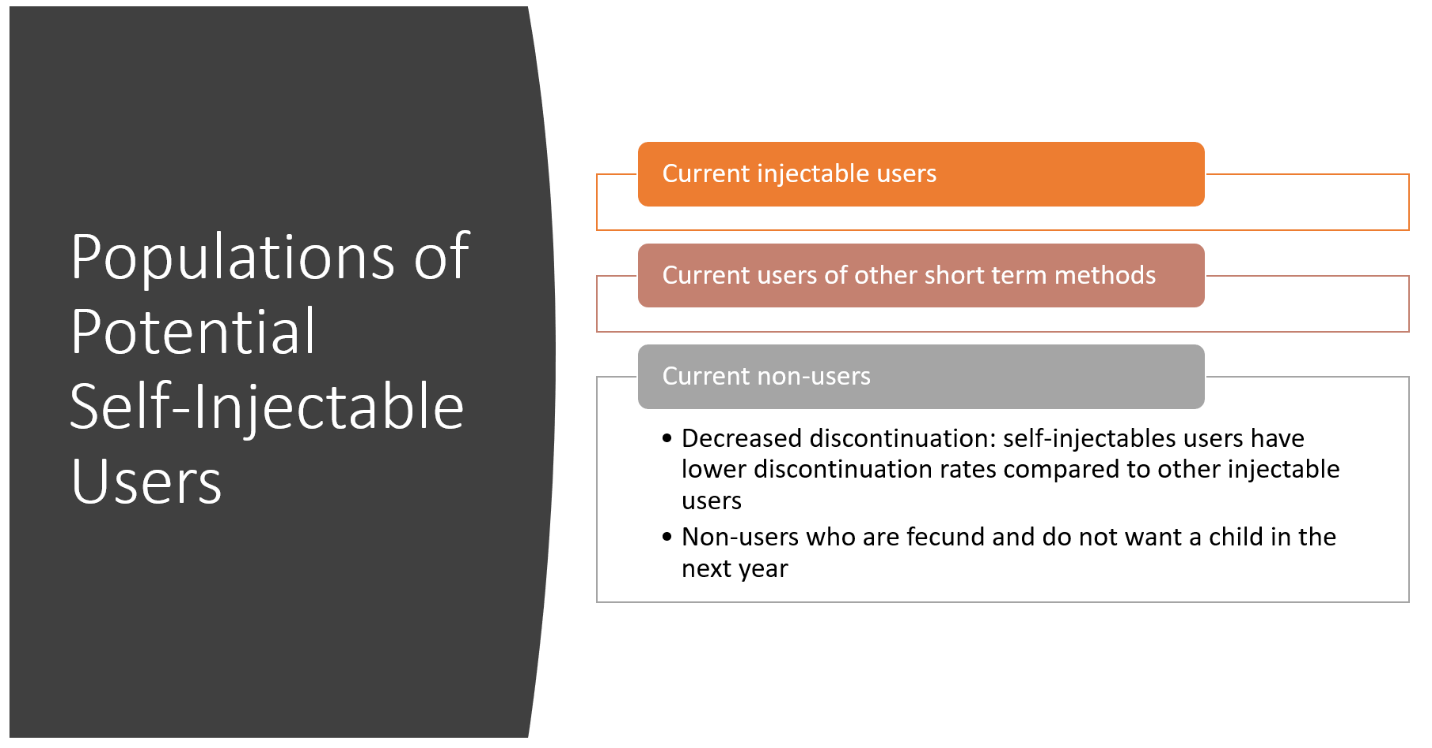
The growth in subcutaneous users is theorized to come from current (intramuscular) injectable users, other short-term method users, and non-users (both from decreased discontinuation of SC and non-users who start using SC). Additional increases will come from these populations when self-injectables become fully available, because of the additional ease of use over provider administered subcutaneous injectables.
Seven parameters are user adjustable. These include:
| Parameters | Default | Notes | Study Links |
|---|---|---|---|
| Proportion of Injectable users that will switch to subcutaneous | 16% | Based on Burkina Faso Pilot. The share of SC user of all injectable users when methods offered in parallel at all health levels | Stout A, Wood S, Barigye G, Kaboré A, Siddo D, Ndione-Colli I. Expanding access to injectable contraception: results from pilot introduction of subcutaneous depot medroxyprogesterone acetate (DMPA-SC) in 4 African countries. Glob Health Sci Pract. 2018;6(1):55-72. https://doi.org/10.9745/GHSP-D-17-00250 |
| Proportion of STM users that will switch to subcutaneous | 8% | There is no available data to directly use for this parameter. The assumption is that the proportion of women switching from other STM will be less than the proportion switching from injectables. There have been studies that ask women their level of interest in switching to SI, but not a study that actually looks at the number that did switch. A 2005 survey in Ediburgh found that 67% of injectable users were interested in switching to self-injection, but only 26% of other women were (this group included some non-users as well). As a placeholder, the assumption is that this parameter is half the amount of injectables users that would switch. | Lakha, Fatim et al. The acceptability of self-administration of subcutaneous Depo-Provera. Contraception, Volume 72, Issue 1, 14 - 18. 2005. https://www.contraceptionjournal.org/article/S0010-7824(05)00014-4/fulltext |
| Proportion of fecund Non-users who do not want a child in the next year that will uptake to SC | 1-5% | There is no available data to directly use for this parameter. The potential pool of non-users that would uptake SC are taken from the DHS. Among women not using, women who are fecund and do not want a child in the next year were considered to be potential users of SC. There is evidence that show fewer side effects of this method and more health providers able to administer is assumed to increase convenience. Khan et all predicted use of self-injectables assumed between 1 and 5% of non-users who intended to use injectables would convert to using self-injectables. Countries are given 1-5% based on their mCPR and injectables as share of the method mix. We constructed linear regressions for mCPR~InjectableShare, and the distance from the predicted line determines their new user uptake parameter. Countries with low injectable use relative to their predicted mCPR are given a higher percentage, as they are assumed to have more room to grow as SC is more like a new method than it is in places where IM injectables are already in high use. | Khan, S. , Grady, B. and Tifft, S. (2015), Estimating demand for a new contraceptive method: Projections for the introduction of Sayana Press. International Journal of Gynecology & Obstetrics, 130: E21-E24. doi:10.1016/j.ijgo.2015.03.020 |
| SI Bonus: Each of the above parameters experiences an increase when SI is at full scale (partial bonus awarded during scale up) | 1-3% | Increase in each of the above parameters when self-injection becomes fully available (partial bonus awarded during scale up) 1-3% We assume that many of the benefits of SC (lower side effects, availability from lower level health care workers, increased availability due to all in one packaging) will be absorbed by the above three parameters. This parameter accounts for the ease of use of self-injection (not having to return to the health center as often). To determine a county's default bonus, we look at the discontinuation (among all methods) because of inconvenience to use- since SI will then have more benefits of SC. We award an additional bonus for countries with high injectable use per given level of mCPR, because while they are predicted to have fewer women uptake, there will be an extra bump in SI that was not seen in SC. | |
| Maximum share of subcutaneous injectables that will be self-injected | 40% | We do not believe that all subcutaneous users will self-inject. First doses will generally be provider administered, and women may be comfortable receiving an injectable from a provider, but not injecting themselves. The 40% default is an estimate based on conversations with PATH's self-injectable team. | |
| Year subcutaneous is at full scale | 2019-2030 | Users choose the year that subcutaneous injectables are out of the pilot stage and fully available. A few countries have years provided by PATH, all other countries we set at 2022 for illustrative purposes. | |
| Year SI is at full scale | 2019-2030 | Users choose the year when self-inject reaches a maximum level. The pathway to SI availability in countries usually starts with SC. Many countries make subcutaneous injectables from providers available earlier than self-injectables. We assume a linear scale up between the year SC become available and SI is at the maximum level. A few countries have years provided by PATH, all other countries we set at 2025 for illustrative purposes. |
The model assumes increased use from decreased discontinuation, the discontinuation rates will decrease with the increased use of SC and SI because of reduced side effects and ease of access. An analysis of 3 randomized, controlled trials found a 1.26 relative risk of 12 month continuation for self-injectable versus intermuscular injectables (Kennedy CE, Yeh PT, Gaffield ML, et al. Self-administration of injectable contraception: a systematic review and meta-analysis. BMJ Global Health 2019;4:e001350. https://gh.bmj.com/content/4/2/e001350). In the model, we use country specific discontinuation rates when available for injectables and other short-term methods and reduce discontinuation by 26%. For example, if 40% of STM users discontinue within one year of use, the number of SC users who come from women who would have discontinued other methods is 0.4 times 0.26. These women come from the original "non-user category."
SwitchingWe assume no switching from long-term methods to subcutaneous injectables.
Constraining GrowthThe model constrains growth based on conditions in the country prior to the introduction of subcutaneous injectables. These constraints include:
- The share of injectables distributed through the public sector. When injectables are already distributed through the public sector, scaling up subcutaneous injectables will be easier. If over 75% of injectables are already distributed through the public sector, we assume no hinderance to growth. If between 50-75% of injectables are distributed through the private sector, we assume 80% of the potential growth can be achieved, and if public sector distribution is less than 50%, we assume 50% of potential growth can be achieved. This scale is only applied to switching from short-term methods to SC and from non-use to SC, we do not assume that distribution channels would change IM to SC use.
- The share of public sector injectables distributed at health centers or community-based distribution. When injectables are mainly accessed through lower levels of services, it will be easier to introduce SC and access will be comparable to current IM injectables. In addition, studies about the introduction of SC focus on these lower levels of service, aligning model assumptions with current introduction procedures. If over 75% of public sector injectables are already distributed through health centers and community-based distribution, we assume no hinderance to growth. If between 50-75% of private sector injectables are distributed through health centers and community based distribution, we assume 90% of the potential growth can be achieved, and if health centers and community based distribution is less than 50%, we assume 75% of potential growth can be achieved. This scale is only applied to switching from short-term methods to SC and from non-use to SC, we do not assume that distribution channels would change IM to SC use.
The constraints to growth are combined multiplicatively. Notes for the selected country are displayed at the bottom of the model.
The user adjusted parameters are annualized, with cumulative growth overtime.
Baseline data for the model comes from several sources:
- Population data for all women 15-49 comes from the United Nation's World Population Prospects 2019.
- Baseline mCPR comes from the 2019 Track20 Global run.
- Baseline distribution of users by type of method comes from the RHSC Community Gap analysis 2020 (Reproductive Health Supplies Coalition. Global Contraceptive Commodity Gap Analysis 2020 (publication forthcoming)).
Code for the model and shiny application can be found at
https://github.com/Track20/SCSIModel